Author: Patrick Woodward
Last week, I attended parts of the Ohio Craft Brewer’s conference, held at the Hyatt Regency in downtown Columbus. For me, the most interesting aspect of the conference was Tom Shellhammer’s keynote address. Shellhammer, the Norwester Professor of Fermentation Science at Oregon State University, is internationally recognized for his research on hop chemistry. His presentation at OCBC focused entirely on the chemistry of dry hopping (adding hops to the beer during conditioning and/or fermentation). As readers of this blog will know, dry hopping is a brewing technique that figures prominently in many styles, most notably American IPAs of every description. Shellhammer’s talk featured three vignettes, each of which had a clear take home message that I attempt to summarize below. I’ve done my best to keep the chemistry at an accessible level.
When More Is Not Always More
The first segment of the talk asked the question, does using larger quantities of dry hops lead to more hop aroma? To answer this question, Shellhammer and his colleagues started with a base pale ale (4.75% abv) that was very lightly hopped (19 IBU), and dry hopped it with whole cone Cascade hops for 24 hours. The variable they chose to explore was the quantity of dry hops, which ranged from 2 g/L to 16 g/L. Translated into units that would be familiar to metrically challenged American homebrewers, the lowest rate of 2 g/L translates to 1.3 ounces per 5 gallon batch (0.5 lbs/bbl), while the highest rate, 16 g/L, is a whopping 10.7 ounces per 5 gallon batch (4 lbs/bbl). The effects of dry hopping on the finished beer were analyzed using both analytical instrumentation and a trained panel of tasters.
The first conclusion is that above 8 g/L the extraction of hop oils and the aroma contributions from those oils are nearly saturated. To use Shellhammer’s language, dry hopping with more than 8 g/L (1.1 oz/gal or 2.1 lbs/bbl) is an inefficient use of raw materials. As a chemistry professor this strikes me as reasonable conclusion. As Tom noted during his talk, the most aromatic components of hop oil have the lowest solubility in water, which limits the amount you can extract. His second finding was more surprising. The oils responsible for citrus aromas saturate earlier, closer to 4 g/L, than the oils responsible for herbal/tea aromas and the compounds that impart bitterness (humulinones and polyphenols). The implications are best summed up in the conclusion of a paper published by Shellhammer and his student Scott LaFontaine:
Adding more hops by static dry-hopping does not simply lead to increased aroma intensity but also changes aroma quality in the finished beer. Dry-hopping rates >8 g/L lead to hop aromas that were more herbal/tea in quality than citrus. To maintain a more balanced hop aroma quality this study suggests using a static dry-hopping rate between 4 and 8 g/L.
How does this compare with dry hopping rates found in practice? I’ve read that Russian River uses approximately 1 lb of dry hops per barrel in Pliny the Elder. While that seemed like a ridiculous amount in the early part of this century, nowadays it doesn’t seem out of the ordinary. Shellhammer’s research would seem to indicate this is still in a range that is useful. Personally, the largest charge of dry hops I’ve used is 4 ounces in a 5 gallon batch, but my buddy Chris Mercerhill once dry hopped with a pound of hops just to see how it would turn out. That experiment, dubbed “The Pounder,” also confirmed the notion more is not always better.

Does Dry Hopping Lead To Bitterness?
Until very recently brewers and brewing scientists attributed hop bitterness almost entirely to isomerized alpha acids that are produced in the boiling wort. The alpha acids found in hops are themselves not particularly bitter and have very little solubility in water, but in the harsh conditions of the boil they are isomerized, which in layman’s terms means that the molecule changes its shape (and polarity) by rearranging its atoms. This change increases both bitterness and solubility. At fermentation temperatures where dry hopping occurs there is not enough heat to isomerize the alpha acids, so the conventional thinking was that dry hopping does not add bitterness. For example, the homebrewing calculator that I use assumes no IBU contribution from dry hopping.
With the recent trend toward intensely fruity IPAs with minimal bitterness, brewers have reduced the hops added to the boil (in some cases to almost nothing), while increasing the dry hopping rates significantly. This unprecedented approach to brewing has led brewers and scientists like Shellhammer to rethink the old assumptions. His research shows that compounds called humulinones and polyphenols can make significant contributions to bitterness in heavily dry-hopped beers. Unlike the isomerized alpha acids these compounds do not need to be boiled to become bitter. Furthermore, because the humulinones are formed when (non-isomerized) alpha acids are oxidized, this effect is more dramatic when using old hops that have partially oxidized. What’s the take home message? Don’t put much stock in the low IBU numbers associated with hazy/juicy NEIPAs and if you do want to minimize bitterness seek out brewers that dry hop with the freshest possible hops.
Hop Creep
The final, and arguably most interesting, segment of the talk explored the phenomenon of hop creep. Never heard of hop creep, don’t feel bad, you would have had plenty of company in the audience at the Ohio Craft Brewer’s Conference. It refers to the process whereby additional fermentation is triggered by dry hopping. Shellhammer described an experiment, which is summarized in the graph below taken from his 2018 article in the Journal of Agricultural and Food Chemistry.
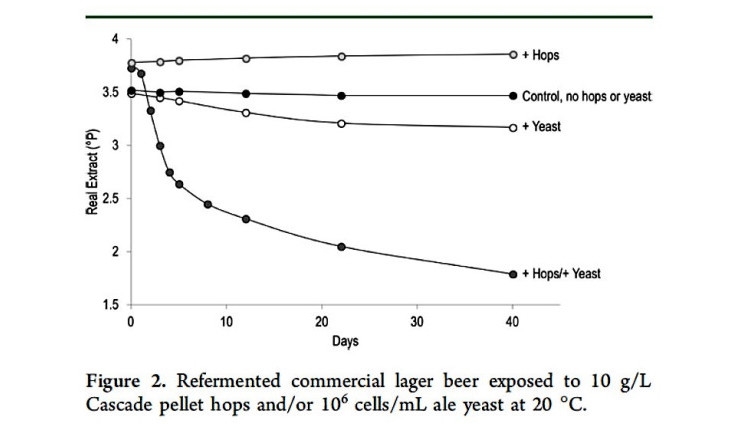
In this study he took four samples of Coors Banquet beer. To one beer he added Cascade hop pellets, to another he added yeast, to a third he added both hops and yeast, while the fourth was not modified. The y-axis of the graph above represents the levels of unfermented sugars in the beer (gravity in brewer’s parlance). The sugar level in the control sample shows almost no change with time, holding steady at 3.5° Plato for 40 days. The beer to which yeast was added shows a modest decrease in sugar levels, perhaps not too surprising, after all Coors doesn’t make the world’s driest beer. The beer to which hops are added shows a slight rise in sugar levels that holds fairly constant for the duration of the experiment. It turns out there are low levels of sugar in hops that can explain this rise. The real shocker is the fourth beer, where both hops and yeast are added. The gravity (sugar levels) of that beer drop by 50% to ~1.7° Plato over the 40-day duration of the experiment!
The important take home point is that additional fermentation only occurs when you add both yeast AND hops. What in God’s green earth could explain this behavior? It turns out there are enzymes in hops that can break down the unfermentable long-chain sugars to simple sugars, much like the enzymes in barley that carry out the same function during the mashing stage. This enzymatic activity doesn’t make much difference if there is no yeast present (sugar is sugar), but in the presence of yeast the newly produced simple sugars are subsequently fermented to ethanol and CO2 . Hop creep is only an issue in dry hopping, because if the hops are added to the boiling or near-boiling wort the high temperatures will denature (destroy) the hop enzymes.
The implications of hop creep can be significant, especially for hazy NEIPAs which often contain suspended yeast and depend on high levels of dry hopping to pack in the hop aromas:
- If the beer has already been packaged the additional CO2 produced during fermentation can lead to exploding cans/bottle bombs.
- A boost in abv will be observed, in the Coors Banquet Beer experiment the abv increased by 1.3%
- Additional diacetyl (an unwanted off-flavor that tastes of imitation butter) is produced. Tests in Shellhammer’s lab showed diacetyl levels rose from 25 ppb to 200 ppb due to the bump in fermentation triggered by dry hopping.
The more unfermented sugar in the beer the more problematic hop creep becomes, which means you’re more likely to experience an exploding DIPA can than a Brut IPA.
Think this is just an academic exercise? Try googling the words “exploding beer cans” and see what you find (or read Bryan Roth’s article on Good Beer Hunting ). After the conference I was speaking with Mark Richards from Land-Grant Brewing and he told me that recently they had to hold off packaging their most recent hazy IPA (Piña Pants) for an extra week because the gravity unexpectedly kept dropping after the second dry hop addition. They were at a loss to explain the behavior until hearing Shellhammer’s talk, after which everything made sense.
This article was originally published on the Pat’s Pints blog, who granted permission for republication on Brülosophy.
| About The Author |
 Pat Woodward has been writing about beer at his Pat’s Pints blog since 2013. He is an enthusiastic homebrewer and recognized BJCP judge. Pat lives in Columbus, Ohio and much of his blog is devoted to stories about the craft beer scene in Ohio, though he was fortunate enough to spend almost a year living in Durham, England where he took every opportunity to explore European breweries and beer styles. Pat and his friend, Mark Richards, from Land-Grant Brewing, can be heard discussing beer with various guests on the Pat’s Pint’s Podcast, available on iTunes and Podbean.
Pat Woodward has been writing about beer at his Pat’s Pints blog since 2013. He is an enthusiastic homebrewer and recognized BJCP judge. Pat lives in Columbus, Ohio and much of his blog is devoted to stories about the craft beer scene in Ohio, though he was fortunate enough to spend almost a year living in Durham, England where he took every opportunity to explore European breweries and beer styles. Pat and his friend, Mark Richards, from Land-Grant Brewing, can be heard discussing beer with various guests on the Pat’s Pint’s Podcast, available on iTunes and Podbean.
If you have any thoughts related to this article, please do not hesitate to share them in the comments section below!
Support Brülosophy In Style!
All designs are available in various colors and sizes on Amazon!
Follow Brülosophy on:
FACEBOOK | TWITTER | INSTAGRAM
If you enjoy this stuff and feel compelled to support Brulosophy.com, please check out the Support page for details on how you can very easily do so. Thanks!












34 thoughts on “The Surprising Science Of Dry Hopping | Lessons From Tom Shellhammer”
Hop creep graph missing?
Terrific ( and useful ) read. Would love to see more super in-depth articles like this.
Read this when pat first posted it. It’s a great informative read. Cool to have a(nother with Malcolm in Ohio) Buckeye putting up Brulosophy content!
Great article! Regarding to the first lesson about dry hops amount, Did the author test that in high ABV beers too? (>10% ABV) And Do you know if that 8g/L recommendation is for total dry hopping? I mean, if one makes double or triple dry hopping, Is it useful to use 8g/L each time?
I read it as total dry hop since it’s talking about the maximum amount of hop oil dissolved in a solution. No matter how you divide it there would still be a maximum limit.
I had a similar question about this. I see @Cory’s point about a total amount that can be dissolved, but it seems like the ethanol content could effect the result. I’m not sure about the differential solubility of these compounds into water vs ethanol or if that difference would be significant at the low levels of alcohol seen in most beer – seems like something to explore though.
I love reading brewing science journal articles, thanks for spreading these studies to a wider audience.
My bigger criticism with a large percentage of these studies is the use of single yeast strain or hop cultivar. We KNOW that there are going to be differences across these different species/strains, yet why isn’t that tested. For example, does Citra saturate at the same rate Cascade does? Does Mosaic lead to more or less hop creep? How about if an English yeast is used (rather than US-05) for either of the above questions?
My thoughts exactly. Different types of hops have verrying amounts of different oils so mosaic might have a different saturation rate than Cascade. The only thing this study is showing me is that I might not need to dry hop as much as I usually do to get the same amount of flavor and aroma. However, those rates quoted above won’t be the same for every type of hop.
As a first study I think it makes sense to limit the variables. Once you can demonstrate that there is actually something to study that is when you go crazy with variations. And practically speaking you are talking about hundreds of thousands of possible combinations of yeast and hops, and that doesn’t include hop combinations. So finding that there is something worth looking at is valuable. Before you can do much realistic testing for creep though you need to get some values for added sugars from hop varieties, and nobody seems to be measuring that right now.
Maybe 20 years ago that kind of logic would fly, but not in this scientific era. I didn’t say every possible permutation either. Perhaps I’m just tired of always seeing Cascade thrown in there, not really representative of the current hop scene.
amen. enough with the cascade jesus.
Awesome. I’d love to see more studies on hopping. One thing about this study is that I believe they used bags to contain the hops. That is also a variable to consider. I’ve sort of settled on 1 oz/gal of T90 pellets for my ipa. It seems to be a good tradeoff (after many trials with many beers!)
the dude abides
Great article, I read it with pleasure. I noticed that cone Cascade hops were used in the first experiment. Since I usually use hop pellets for dry hopping I am wondering how to transform the recommended hop quantities from cones to pellets. Can anyone help?
I would love to see a longer and deeper look into the hop creep. The idea to keep more sugars in the beer and to minimize the diacetyl intrigues me. Maybe a thread started on one of the brew forums?
Add something to kill of the yeast before a dry hop? I don’t like this idea.
Cold crash before dry hop? The cold will keep the yeast from reacting with the sugars. My guess the alpha extraction from the hops will fall dramatically.
Dry hop at room temp and start chilling the beer?
I know some breweries that dry-hop at 17C / 62F when the beer gravity is about 1.030, never asked on how much it changed the extract, I can imagine that since fermentation is active it will get rid of the diacetyl.
I usually dry hop after fermentation at 13C / 55F, which stops yeast activity but still extracts aroma, never had diacetyl on my beers.
Great article, I remember reading this when it first hit the internets a few weeks ago. One thing bit that I am not fully clear on – is if the suggested rates (roughly 2.5 to 5 ounces per 5 gallon batch) is the total dry hop charge suggestion, or the per charge? If I am doing a double dry hopped beer – I may be tossing in 3-4 ounces per charge. I am unclear if that means I am overdoing the overall hops and getting nothing out of it, besides a hole in my wallet, or if I am right on the mark?
I read it as total dry hop since it’s talking about the maximum amount of hop oil dissolved in a solution. No matter how you divide it there would still be that maximum limit. It would seem to suggest if you DDH to keep each charge to around 2.5oz. As others have mentioned it would likely be hop dependent as well since different hops have different concentrations and total amount of oils.
Hop creep is for real. My latest IPA was 1.009 before I dry hopped and the FG after two days was 1.004.
I wonder about the hop saturation when I keg hop. Because some of the hop aroma is fading and yet there is the hops sitting in there. I imagine the hops in the keg resaturate the aroma.
Yeah! I was waiting to see someone say this. I got just about that exact same drop after my second dry hop. FG was 1.004. I had to scribble that reading into the margin of my brew journal.
So this finding re: hop creep would seem to confirm that “biotransformation” is real and measurable.
This is a great article. I’m pretty sure hop creep just blew my mind. Also, I’m calling dibs on that for a band name.
Excellent summary, thank you.
At what temp do the hop enzymes break down simple sugars? If you cooled your wort post-boil down to, say, 160°F (at/near pasteurization temps) and added your flameout hops there, would you get the benefits of the flavor boost AND a breakdown of complex sugars that would make the wort more fermentable and more likely to dry out to a lower FG?
The requirement for hops and yeast make sense since he was using Coors, who filter out the yeast before packaging. In homebrew where you still have yeast in the finished beer I’m pretty sure you would get the creep by just adding hops.
In regards to hop creep, perhaps the dry hop addition creates enough nucleation points to release CO2 which gently agitates enough of the yeast to coax them into further fermentation? Just a thought.
Excellent article. Can the pasteurization process also be useful to drive the denaturation of hop enzymes?
Great read! With hop creep and exploding cans/bottles in mind, would 40 days conditioning be the recommended time for a heavily dry hopped beer? Cheers!
…Looking at the hop creep graph, 20 days is looking pretty good!?
I was waiting for a comment in the article that after adding 16 g/l whole hops to the beer, that no beer was left in the container after removing the hop matter.
Regarding the “More is not better” theme, there seems to be a missleading conclusion to make. First of all, they only look at a 4.75% ABV pale ale. What happens when you brew a 8% NEIPA. You almost double the ratio of ethanol in the solution, which could potentially impact the hop oil solubility. I’m not saying i “know” this, but I think it would be unwise to dismiss this factor. They also kind of misleads you with that graph. If you look at “overall” aroma, the points are actually increasing but they have draws a “function” that have a maximum before the last point, so it seems to be decreasing after 1600 g/hL, but there are not any data that would suggest this. It could be increasing linearly after 1600g/hL for all we know.
Great article, thank you.
The dry hop bitterness confirms my own experiment. I once discovered some old bottles of homebrewed IPA which had lost much bitterness and hop flavor. I carefully added small measured amounts of hops to each bottle and recapped. The beer gained both bitterness and flavor.
This finally gives an explanation as to why my heavily dry hopped pale es always ended up overcarbing and exploding when bottling
Awesome article!!! one question though… any idea on the time the additional enzymes take to convert complex to simple sugars? I´d like to understand the timing around these processes… the chart posted is great but for a dry hopping scenario I´d like to understand how long would it take for enzymes to act and afterwards, for yeast to consume and diacetyl to be re-absorbed. I know a lot of variables in place but it´d be good to have an idea. Thanks!