Author: Jake Huolihan
When fermented beer comes into contact with oxygen, reactions begin to occur that ultimately cause the beer to take on undesirable characteristics and thus reduce its shelf-life. Modern brewers employ a variety of methods to keep cold-side oxygen exposure at a minimum, with the goal being to ensure the beer tastes fresh for as long as possible.
The point in the brewing process where cold-side oxidation is most likely to occur is during packaging, as there exist many vectors for oxygen exposure when moving beer from one place to another. To mitigate this risk, brewers often use carbon dioxide (CO2) to push the beer out of a fermenter into a keg that has been purged of oxygen with CO2.
With so many past xBmts demonstrating the negative impact cold-side oxygen exposure can have on beer, I was surprised with my inability to reliably tell apart a pale lager pressure transferred to a purged keg from one that was racked using gravity into a non-purged keg. Interested to see how this would play out with a different style, I tested it out again on a British Strong Bitter.
| PURPOSE |
To evaluate the differences between a Strong Bitter pressure transferred to a keg purged with CO2 and one transferred to a non-purged and open keg.
| METHODS |
For this xBmt, I went with a simple Strong Bitter recipe, as I was curious how one of my favorite styles would react to these packaging differences.
Barely There
Recipe Details
| Batch Size | Boil Time | IBU | SRM | Est. OG | Est. FG | ABV |
|---|---|---|---|---|---|---|
| 5.5 gal | 60 min | 42.7 | 8.8 SRM | 1.051 | 1.015 | 4.73 % |
| Actuals | 1.051 | 1.015 | 4.73 % | |||
Fermentables
| Name | Amount | % |
|---|---|---|
| English Pale Ale | 11 lbs | 89.8 |
| Cara Ruby | 8 oz | 4.08 |
| Malted Oats | 8 oz | 4.08 |
| Double Honey | 4 oz | 2.04 |
Hops
| Name | Amount | Time | Use | Form | Alpha % |
|---|---|---|---|---|---|
| Loral | 32 g | 30 min | Boil | Pellet | 11.5 |
| East Kent Goldings (EKG) | 55 g | 10 min | Boil | Pellet | 5 |
Yeast
| Name | Lab | Attenuation | Temperature |
|---|---|---|---|
| House (A01) | Imperial Yeast | 75% | 32°F - 32°F |
Notes
| Water Profile: Ca 91 | Mg 0 | Na 8 | SO4 149 | Cl 55 |
Download
| Download this recipe's BeerXML file |
I started my brew day by collecting RO water, adjusting it to my desired profile, then flipping the switch on my controller to get it heating up, after which I weighed out and milled the grain.
With the water properly heated, I mashed in then checked to ensure it was at my target temperature.
While waiting on the mash, I prepared the kettle hop additions.
Once the 60 minute mash step was complete, I collected the sweet wort in my kettle and brought it to a boil, adding hops as stated in the recipe.
When the 30 minute boil was finished, I quickly chilled the wort with my IC.
A refractometer reading showed the wort was right at my intended OG.
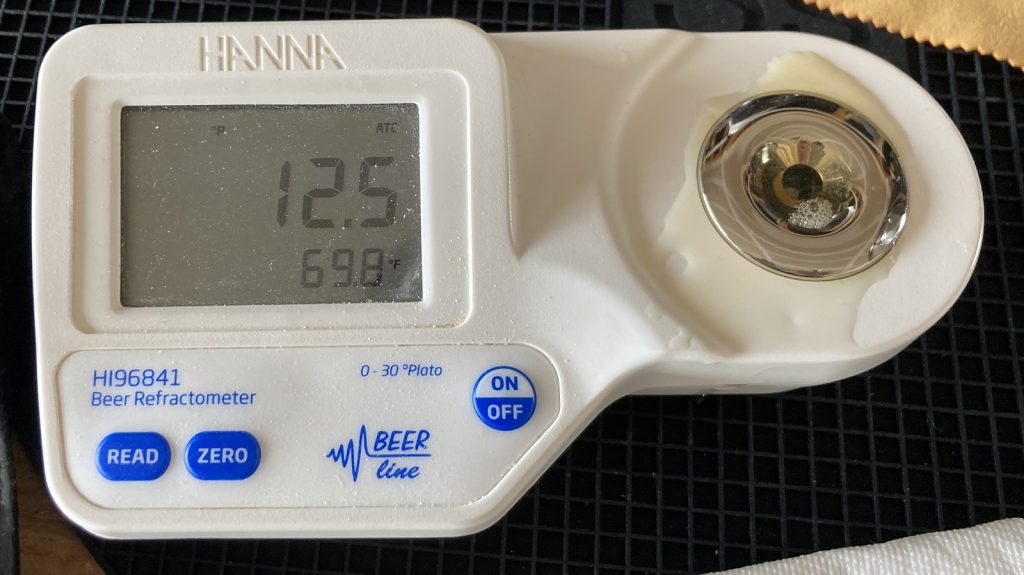
After evenly splitting the wort between two fermentation vessels, I used my glycol unit to finish chilling them to my desired fermentation temperature of 68°F/20°C before pitching a single pouch of Imperial Yeast A01 House into each.
After a couple weeks, fermentation activity had dwindled in both beers so I took hydrometer measurements showing they were at the same FG.

I left the beers alone for another week before proceeding with packaging. While one beer was racked via gravity to a non-purged keg, the other was pushed from the fermenter with CO2 into a purged keg.
The filled kegs were placed in my keezer and left to condition for 4 weeks before they were ready for evaluation.

| RESULTS |
Due to social distancing practices as a result of the COVID-19 pandemic, data for this xBmt was unable to be collected in our typical manner. As such, temporary adaptations were made involving the author completing multiple semi-blind triangle tests in as unbiased a way as possible.
Utilizing 4 opaque cups of the same color where 2 were inconspicuously marked, one set was filled with the beer transferred under pressure to a purged keg while the other set was filled with the beer racked via gravity to a non-purged keg. For each triangle test, 3 of the 4 cups were indiscriminately selected, thus randomizing which beer was the unique sample for each trial. Following each attempt, I noted whether I was correct in identifying the unique sample. Out of the 10 semi-blind triangle tests I completed, I needed to identify the unique sample at least 7 times (p<0.05) in order to reach statistical significance. In the end, I correctly identified the unique sample just 2 times (p=0.90), indicating my inability to reliably distinguish a Strong Bitter that was beer transferred under pressure to a purged keg from one that was racked via gravity to a non-purged keg.
These beers tasted identical to me, which I didn’t expect based on past cold-side oxidation xBmts. Both were very tasty examples of Strong Bitter with a nice malt character that was balanced by the English hops and subtle esters from the English yeast.
| DISCUSSION |
Cold-side oxidation is an issue that commercial brewers rightfully concern themselves with, as it’s well known that exposure to oxygen once fermentation is complete can rapidly reduce shelf-life. Of the various methods employed to reduce the chances of cold-side oxidation, a common one involves using CO2 to push beer from the fermenter into a keg that has been purged of oxygen with CO2. My inability to reliably distinguish a Strong Bitter that was pressure transferred to a purged keg from one that was racked via gravity to a non-purged keg suggests any differences between these beers were minimal enough as to be imperceptible by me.
This is now the second xBmt I’ve performed using our Covid-19 protocol where I was unable to tell beers packaged in these disparate ways apart, though a similar xBmt where the style was NEIPA returned significant results. While we’re unable to make any claims of certainty based on these findings, they add to the plausibility of the idea that something about NEIPA makes it more sensitive to oxygen than other styles.
I’ve not always been as concerned with cold-side oxidation as I am today, which was admittedly influenced by the rapid deterioration I observed in hazy IPA exposed to oxygen post-fermentation. Even though I’ve had good results in past batches of non-hoppy styles racked into non-purged kegs, I have no plans to change my more neurotic packaging process of pressure transferring to purged kegs. To me, it’s a better-safe-than-sorry thing that requires minimal additional effort.
If you have any thoughts about this xBmt, please do not hesitate to share in the comments section below!
Support Brülosophy In Style!
All designs are available in various colors and sizes on Amazon!
Follow Brülosophy on:
FACEBOOK | TWITTER | INSTAGRAM
If you enjoy this stuff and feel compelled to support Brulosophy.com, please check out the Support page for details on how you can very easily do so. Thanks!




















23 thoughts on “exBEERiment | Cold-Side Oxidation: Impact Of Transferring a British Bitter To A Non-Purged Keg”
If this exp was looking at the potential impact of oxidation, why was Brewtan-B invluded in this recipe? I don’t recall it being regularly in other Brulosophy recipes. According to the manufacturer, Brewtan-B is supposed to reduce oxidation in the final beer. Even your own exp showed a significant result. This seems like an odd choice to begin including it in this study.
Adam, exactly my thoughts!! Maybe try a brewtan vs without…..
Must have been a copy/paste error. Looks like article was edited to remove it from recipe.
Where do you see Brewtan-B?
Yup, Brewtan is a powerful antioxidant and would have definitely affected this experiment. It would have scavenged and removed oxidative metals long before the open-air racking, so the staling reactions would take roughly 4x longer to occur. The amount of oxidation in the Brewtan 4-week sample should be roughly equal to 1 week without Brewtan.
I am confused, I do not see any mention of Brewtan in the write-up…??
It appears that it has been edited out of the recipe now. The original version had Brewtan-B listed in the ingredients. I’m hoping that means it was a typo in the original version. Hopefully someone from the team could verify.
4 weeks doesn’t allow any real impact of oxidation. Beers like this will take time to oxidize in the container and should change the shelf life of the product 3 months or longer out. Can you revisit these beers later?
I find it a bit pathetic that you make these comparisons, find no significant impact but conclude that despite not being able to distinguish any difference you will continue to purge your kegs or whatever you were testing as if you don’t trust your own results.
Perhaps you should try to put several tests for, say cold-side oxidation, that yielded negative results, together into one test and see if there’s a significant difference.
I bet that would create some discussion.
If they do, you can eliminate the variables one by one.
I would be interesting in those results.
“Pathetic” seems harsh. But I do find it amusing.
Neil: Exactly! Isn’t it like Mythbusters? See how far you have to go to actually notice the results.
It is getting a bit tiresome having all these experiments that use subjective tastebuds to measure very subtle variables. Brulosophy needs to get back to its roots.
Töm
I do lots of things that probably don’t matter, too. The gods get pissed when you get lazy and cut corners. Besides, pressure transferring is cool AF.
“Besides, pressure transferring is cool AF” – 100% with you Mr. Fisher. I open ferment my hefe’s for 72 hours but close ferment and pressure transfer everything else.
I think you’ve got to see the limitations of this kind of sensory experiments. Although this was just one tester, usually it’s done in groups. Now if you have a group of 20 people and only 5 pick the correct sample, although not significant outcome this does not mean the 10 don’t notice the difference. They could have had chance on their side or they could have super senses (or any mix). If the latter were true, in the above case that’s potentially (up to) 50% of your audience which is still something worth considering.
If I’m giving a beer to someone I can guarantee they won’t perceive exactly what I perceive so regardless of what I taste I’m going to follow best practice theory that can only improve a beer (with the theory based upon comparing the outcome of two extremes). I figure there is going to be someone that has a better palette than me or is more sensitive to a particular taste
Sorry, I meant to say 10 pick the right sample*
To me, what would be interesting would be what the beers would taste like after lets say a month? Would the presence of oxygen change the flavor profile after a period of time
Dave, it says that the beers were left to condition for 4weeks aka a month before evaluation. It would be interesting to re visit after another month or 8-10 total weeks in keg and see if there is any perceptible change in them tho…
Are these purged kegs ones that had star san and then pushed out with CO2 to replace the star san with CO2, or were the kegs empty, filled with CO2 to a certain pressure, then burped a number of times to try and force the air out? I’ve had more success staving off oxidation with NEIPAs by filling the keg with Star San, then pushing out the liquid to leave CO2 behind prior to transferring my beer into the keg.
What is double honey?
Specialty malt from Root Shoot Malting
https://lake-hollow-homestead.square.site/product/double-honey-malt/62?cp=true&sa=false&sbp=false&q=false&category_id=6#
Both of these samples were kept cold for the 4 week rest, resulting in the higher disolved oxygen in the non purged keg to degrade the beer at a much slower rate.
You’ve already done the most important/effective measure against disolved oxygen’s negative effects by keeping it cold.
What was the carbonation process? How much headspace( if any) did either keg have?
I just finished listening to the EP 197 water chemistry adjustment episode and have a thought. Chemistry was never one of my strengths, so I’m sure someone with a better chemistry background than mine could poke this theory full of holes. I’m probably going to have to re-listen to the podcast when I can pay more attention to it, as opposed to background entertainment.
I wonder if the severity of cold side oxidation off-flavours is perhaps driven, in part, by water chemistry. Could be water straight from the tap, or adjusted by the brewer.
As an example, lets say someone adds a whack of table salt because they want chloride ions for whatever reaction desires chloride ions, they will have sodium ions that need to go somewhere.
This is pure, partially educated speculation on my part, but if there is a lot of free sodium that hypothetically makes “methyl-ethyl-bad stuff” in the presence of oxygen, that’ll be highly susceptible to oxidation. But if there is minimal free sodium, theoretically you wouldn’t get much “methyl-ethyl-bad stuff,” regardless of how much oxygen is present.
You should try it with a more oxygen sensitive beer. And try a different test method.