This xBmt was completed by a member of The Brü Club as a part of The Brü Club xBmt Series in collaboration with Brülosophy. While members who choose to participate in this series generally take inspiration from Brülosophy, the bulk of design, writing, and editing is handled by members unless otherwise specified. Articles featured on Brulosophy.com are selected by The Brü Club leadership prior to being submitted for publication. Visit The Brü Club website for more information on this series.
Author: Cade Jobe
I’ve been trying to dial in a go-to Kölsch recipe for the hot Texas summers, which in my opinion should have a very light bready flavor with a medium-low bitterness and leave the palate with a crisp, vinous quality that accentuates the dryness. Previous versions have yielded comments on competition scoresheets suggesting previous batches have had a slight tang, or what one judge described as an “apple-like bitter ester.” I brew with local tap water and make adjustments to achieve specific target profiles. Due to my tap water being fairly alkaline, I end up using a decent amount of lactic acid to ensure the mash pH is in the appropriate range.
I’ve read that tasters are able to identify lactic acid in beer at levels above 1.5 mL/gal, and Martin Brungard offers the following on the Water Knowledge tab of his Bru’n Water Spreadsheet:
Lactic acid is readily available for brewing use, but it can produce a distinctive ‘tang’ in the flavor profile at high concentrations. Phosphoric Acid can also be used and it has little flavor effect since this acid is similar to the malt acids produced through mashing.
While contributor Malcolm Frazer’s xBmt comparing lactic acid to phosophoric acid returned non-significant results, I was curious to see if this might be the reason for the tangy characteristics perceived in my Kölsch and decided to test it out for myself!
| PURPOSE |
To evaluate the differences between beers made using either lactic acid or phosphoric acid to mash pH adjustment.
| METHODS |
The recipe for this xBmt was an iteration of one I’ve been working for awhile that’s almost, but not quite, there.
Fortitude Kölsch
Recipe Details
| Batch Size | Boil Time | IBU | SRM | Est. OG | Est. FG | ABV |
|---|---|---|---|---|---|---|
| 5.5 gal | 60 min | 20.3 IBUs | 3.0 SRM | 1.049 | 1.009 | 5.3 % |
| Actuals | 1.049 | 1.008 | 5.4 % | |||
Fermentables
| Name | Amount | % |
|---|---|---|
| Pilsner (Weyermann) | 9.5 lbs | 95 |
| Wheat Malt, Pale (Weyermann) | 8 oz | 5 |
Hops
| Name | Amount | Time | Use | Form | Alpha % |
|---|---|---|---|---|---|
| Hallertau | 28 g | 60 min | Boil | Pellet | 3.6 |
| Tettnang | 16 g | 60 min | Boil | Pellet | 3.6 |
| Liberty | 14 g | 5 min | Boil | Pellet | 4.5 |
Yeast
| Name | Lab | Attenuation | Temperature |
|---|---|---|---|
| German Ale/Kolsch (WLP029) | White Labs | 75% | 65°F - 69°F |
Notes
| Water Profile: Ca 94 | Mg 17 | Na 18 | SO4 150 | Cl 50 |
Download
| Download this recipe's BeerXML file |
I started my #BrüDay by collecting the brewing water for the batch treated with lactic acid, as the beers were brewed consecutively but otherwise treated identically with the exception of the variable.
While the water for the first batch was heating up, I quickly tested it with a BrewLab kit to confirm the starting profile. Next, I used the Bru’n Water Spreadsheet to determine the additions needed to achieve my desired profile, adjusting both batches with either lactic or phosphoric acid to reach the same mash pH.
Next, I weighed out and milled the grains.
Once the water for each batch was at my target strike temperature, I mashed in then immediately made the respective acid addition.
Measurements taken at 15 minutes into each mash confirmed both had settled at a similar pH.

After each 60 minute mash, I sparged to collect identical volumes of wort that were subsequently brought to a boil. At the end of each 60 minute boil, I used my immersion chiller to quickly cool each wort.
Equal amounts of wort from each batch were transferred to sanitized Brew Buckets and set in my fermentation chamber to finish chilling.
Refractometer readings showed both batches hit the same 1.049 OG target. Once the worts had stabilized at my desired temperature of 60°F/16°C, the yeast was pitched. After 14 days of fermentation, hydrometer measurements revealed both beers had reached the same 1.008 FG. I cold crashed the beers overnight and fined with BioFine Clear before transferring each into CO2 flushed kegs. The filled kegs were burst carbonated then allowed to cold condition for 2 weeks before they were ready to serve.

| RESULTS |
A total of 23 people of varying levels of experience participated in this xBmt. Each participant was served 1 sample of the beer where lactic acid was used for mash pH adjustment and 2 samples of the beer acidified with phosphoric acid in different colored opaque cups then asked to identify the unique sample. In order to achieve statistical significance at this sample size, 12 tasters (p<0.05) would have had to accurately identify the odd-beer-out, and that’s exactly the number that did (p=0.048), indicating participants in this xBmt were able to reliably distinguish a beer acidified with lactic acid during the mash from one acidified with phosphoric acid during the mash.
The 12 participants who made the accurate selection on the triangle test were instructed to complete a brief preference survey comparing only the beers that were different. A total of 9 tasters reported preferring the beer made with phosphoric acid, 1 liked the beer made with lactic acid, and 2 people did not report a preference.
My Impressions: The night before serving the samples at a homebrew club meeting, my wife (who is no longer pregnant and can drink now! Yay!) and I took two semi-blind triangles tests. While we both chose correctly, I admittedly guessed on my second attempt. Several days after collecting the data, we both attempted a final test and failed. The beers were much closer in overall character than they were different, at least to my palate.
| DISCUSSION |
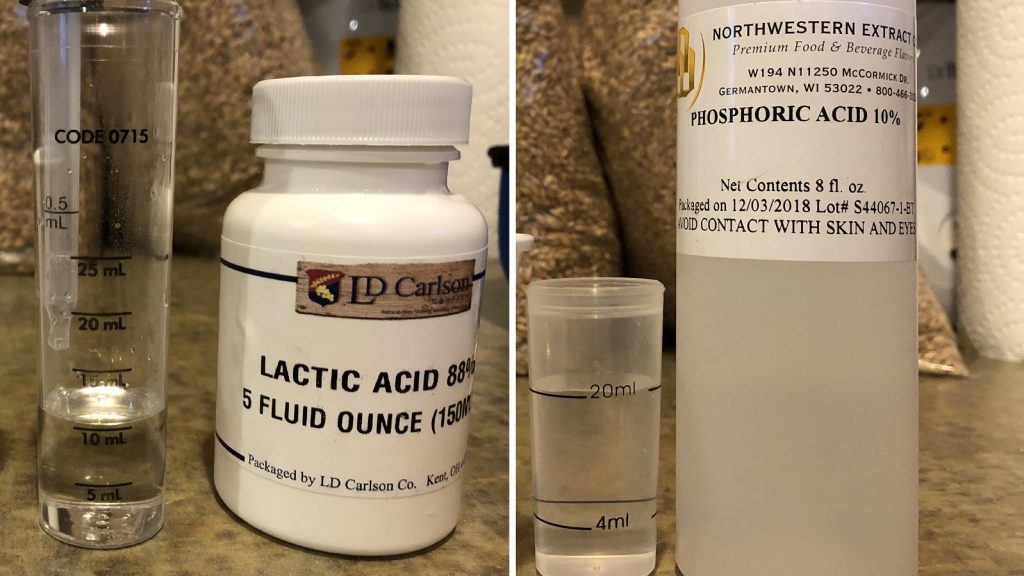
While Malcom’s original xBmt comparing the use of lactic and phosphoric acid to adjust mash pH failed to reach significance, such was not the case in my own comparison, as tasters were able to reliably tell apart beers made using either acid. One of the more obvious differences between the xBmts had to do with total amount of acid used– whereas Malcolm used 7.2 mL of 88% lactic acid and 4.1 mL of 75% phosphoric acid for a 5.5 gallon batch, I used 10.4 mL of 88% lactic acid and 108 mL of 10% phosphoric acid to achieve a similar mash pH. It’s possible this higher concentration of lactic acid helps to explain the discrepant findings, which would support the notion that lactic acid imparts a perceptibly different character than phosphoric acid when higher amounts are used.
In conversations with tasters following data collection, several of those who were correct on the triangle test said the beer made with phosphoric acid had more back-end flavor, which made it more preferable to them. Interestingly, while a couple participants noted perceiving certain common off-flavors including acetaldehyde and diacetyl, not one described a “tangy” character.
I found it interesting that 9 of the 12 correct tasters preferred the beer made with phosphoric acid. I don’t know what I don’t know, so I’m unable to come up with a satisfactory explanation for their preference or the difference in these beers. As for me, I’m not done tweaking my Kölsch recipe and plan to use phosophoric acid for mash pH adjustment in my next few batches, which I’ll enter in competitions and let subjective judging challenge the objective significant results of this experiment!
 Cade Jobe is a Certified Cicerone and Certified Beer Judge (BJCP) living in Austin, Texas. He is a member of the Austin ZEALOTS who loves brewing and spending time with his wife, son, and two dogs named Juno and Jasper. Follow Cade on Instagram, Twitter, and UnTappd.
Cade Jobe is a Certified Cicerone and Certified Beer Judge (BJCP) living in Austin, Texas. He is a member of the Austin ZEALOTS who loves brewing and spending time with his wife, son, and two dogs named Juno and Jasper. Follow Cade on Instagram, Twitter, and UnTappd.
If you have any thoughts about this xBmt, please do not hesitate to share in the comments section below!
Support Brülosophy In Style!
All designs are available in various colors and sizes on Amazon!
Follow Brülosophy on:
FACEBOOK | TWITTER | INSTAGRAM
If you enjoy this stuff and feel compelled to support Brulosophy.com, please check out the Support page for details on how you can very easily do so. Thanks!
















44 thoughts on “The Brü Club xBmt Series | Water Chemistry: Lactic Acid vs. Phosphoric Acid for Mash pH Adjustment In A Kölsch”
Good exbeeriment. I would like to know the amounts of each acid used, as these are the variable so they should be detailed near the top. Lactic is mentioned in the discussion, but how much Phosphoric?
I’ve stopped using Lactic myself as I have found my beers with it to have an undesirable taste. Upon removing Lactic the undesirable taste went away; maybe I’m just sensitive to it and I always use distilled water so acid additions are not that much. Thanks!
There is a picture with the amounts being measured…
Good point. I just did a quick edit to clarify. Cade used 108 mL of 10% phosphoric acid.
108 ml of 10% Phosphoric, 10.4 ml 88% lactic. That’s an interesting note about an “undesirable” taste. That’s similar to what some of the tasters said, though they couched it more as “less desirable” than “undesirable.”
What taste? Some of my beers I use lactic with will have a almost puckering dryness at the end. Astringent as some would call it. I don’t over mill my grain either. Was always wondering if that was lactic causing that.
While not directly connected to the variables tested here, have you tried different yeast strains for your Kolsch? You mentioned vinous quality which I think the Wyeast strain is known for (at least more so than the WL strain).
https://brulosophy.com/2015/08/31/yeast-comparison-white-labs-wlp029-vs-wyeast-wy2565-xbmt-results/
Good idea! I also want to try out Imperial’s Dieter.
In my own home brewery, I have always preferred phosphoric. I don’t have quantifiable evidence why this is, but know my beers tasted better to me with phosphoric.
I’m excited to see how the next few batches turn out with phosphoric.
Suggest you either filter you home water or use filtered and mineral cleared water that you can get at stores.
Thanks for the suggestion.
I’ve used acetic acid for years along with mineral adjustments.
I’d love to see that thrown into the hat here. I’ve never had a complaint implying vinegar in on finished beers. 🍻
I think you need to update your bio at the bottom of this article. And congrats!
That’s my bad. Thanks for the heads up!
In our Advanced Home Brewing Class, Water Module – we mix two taste samples. A few ounces of distilled water acidified to a pH of 5.2-5.5 with each type of acid. Then you can clearly taste the difference. Most people prefer the neutral taste of phosphoric. So that’s what I use. Got the tip seven years ago from Gordon Strong’s first book Brewing Better Beer, page 147. Cheers!
That’s in isolation though. In a finished beer is quite possibly a different story (though this experiment would suggest otherwise). Hell, in a mash might be a different story. Don’t phosphoric acid and CaCl2 react to form calcium phosphate and HCl?
What about Acetic Acid?
Hmm. No thanks.
Is there a reason? I mean that was my purpose. I’ve been using higher strength acetic acid for years and have had no negative reviews. So is it worth it for me to attempt switching to another acid that I can’t just buy from nearly any store?
Is it more or less calcium sparing than phosphoric? Are there actual negative impacts in the chemistry or the perceived flavors?
My experience on the drinking end is crowds of friends blowing my kegs regularly and I live in Washington where these folks all go to local breweries regularly.
I’m just super curious as it seems to be an uncommon practice.
Love ya guys.
Oh dude, I honestly thought you were making a joke! I’m sorry. Acetic acid, aka vinegar, is something I don’t want in my beer at all. It’s entirely possible that using small amounts of lab grade acetic to adjust mash pH won’t have a flavor impact, it’s just something I wasn’t aware people were doing.
I know of a few people who regularly use ascorbic acid, aka vitamin C, for pH adjustment and have good results.
Sorry, maybe I should have just sent an email.
My wife buys cleaning strength acetic acid (6% I believe) for cleaning and some other things. I initially tried it years ago as a last resort to bring my PH down.
Worked great and once I started testing (vice just calculating) PH I started using it more regularly. Now I use it exclusively. I’m not necessarily advocating its use generally speaking, only that I’ve been very successful in my recipe development using vinegar and BRU’n Water.
Plus I’m never wanting for it, I can steal it from my wife’s stores anytime.
No worries, cheers!
Second experiment in I think a month acheiving exactly the right number to achieve significance. Just saying.
Just saying… what?
If it was my lab, I’d start keeping a closer eye on things and maybe more diligently reading student’s notebooks every night after they went home for the day.
Ok.
I had a discussion about this same topic with Malcolm Frazier about a strange flavor I was getting in some of my beers where I used lactic. I mentioned that when trying to nail down the issue I smell my lactic and it reminded of the off flavor. He said some times you will get lactic acid that just is “off” and suggested trying phosphoric. I’ve haven’t used lactic since that talk and have not experienced the same “tang”. Not certain that was the issue but it appears to be at least a contributor. Great work on the xbmt!
Really?
I noticed you said nobody noted tangy flavor but several mentioned acetaldehyde. For some people, they perceive low level acetaldehyde as a tangy taste, so they may have simply mistaken the tang of low level lactic acid for acetaldehyde.
Just to clarify: only 1 mentioned acetaldehyde; not several. I agree with the rest of the comment, though. It’s certainly possible the taster identified the lactic flavor and described it as acetaldehyde.
Really interesting stuff here! I exclusively use 88% Lactic acid for all mash adjustments and pre-acidifying kettle sours.
To me, the phosphoric acid has a metallic flavor. So crazy how different all of our tastes can be!
I should note: I only do acid mods for pale beers. But *all* of my beers get either Gypsum or Calcium Chloride …. or both. I know for damned sure those make a big difference.
In the UK we use something called Brupak’s CRS (carbonate reducing solution) to remove bicarbonate from water with high alkalinity and so acidify the mash. It’s a blend of hydrochloric and sulphuric acids. The price you pay is higher sulphate and chloride levels, but you can keep an eye on their levels with a good water treatment calculator. I haven’t encountered any off flavours using CRS and a bit of extra sulphate doesn’t bother me, but I’d be interested to know how CRS compares to other acids. It’s odd that it doesn’t seem to be available to US brewers.
Just a heads up… I believe that the numbers do not add up in the paragraph: “The 12 participants who made the accurate selection on the triangle test were instructed to complete a brief preference survey comparing only the beers that were different. A total of 9 tasters reported preferring the beer made with phosphoric acid, 1 liked the beer made with lactic acid, and 3 people did not report a preference.”
Cheers
Just double checked and saw that 2 people reported no difference, not 3. Thanks for the heads up.
Is there a reason you used 10% phosphoric instead of 88%? Thanks so much for another great post!
10% is the only concentration available from my LHBS.
Love this xBmt. I stopped using acidulated malt based on the first test by Malcom and some additional information gleaned from a few articles I’ve read. My beer has never tasted better. The “apple-like bitter ester” flavor Cade refers to was in all of my light-colored beers, regardless of the yeast, water makeup, or pH. Get some 85% phosphoric acid, a really good syringe, and Bru’n Water and you are pretty much set. Downside, I guess I’m no longer brewing under the Reinheitsgebot. Also, isn’t Star San mainly phosphoric acid?
Yea, Star San is 50% phosphoric acid (75%) and 15% dodecylbenzene sulfonic acid.
The other 35% is a secret.
Thanks! I’m interested to see how the next few batches come out. If I remember, I’ll post some comments here from score sheets.
Quick process question. Any reason the acid was added at mash in? I usually add salts and acid prior to mash in so i can stir/disolve things well before mash in. just curious what others view as the best practice
Just to complicate the discussion – I use tartaric acid to get my mash ph in line. I started using it to dissolve calcium scale out of kitchen appliances so that I wouldn’t have to deal with rinsing out the strong smell of acetic acid. It seemed to make sense to use it in my mash instead of lactic to avoid the lactic flavor. I have been using it for a year or so and it seems OK, but I never did a side by side test.
Have you tested Acid malt vs acids? That would be very interesting to see. The German breweries who brew according to the old beer cleanliness law is using acid malt to comply. I have also seen RO water in use mixed in to lower pH. That would also be a very interesting test, that would in fact not add any acid, just remove buffer to lower the pH…
Acidulated Malt vs. Phosphoric Acid xBmt
I just heard the podcast to this xBmt and Cade, saying his wife detected a certain “upness” in the phosphoric acid beer, reminded me of the fact that there is phosphoric acid in Coca Cola…