This xBmt was completed by a member of The Brü Club as a part of The Brü Club xBmt Series in collaboration with Brülosophy. While members who choose to participate in this series generally take inspiration from Brülosophy, the bulk of design, writing, and editing is handled by members. Articles featured on Brulosophy.com are selected by The Brü Club leadership prior to being submitted for publication. Visit The Brü Club website for more information on this series.
Author: Scott Mendes
Count to 3.
One Mississippi, two Mississippi, three…
Is it everything you were expecting? Too bitter? Too sweet? Flavorless? What exactly is that flavor? Is it balanced enough? How’s the mouthfeel? Oh, that aroma! Do I want another? What should I adjust? Is it perfect?
…Mississippi.
This is the amount of time it likely took your brain to decide if that prickly hop and malt flavored water you just took a sip of was good or not. With so many aspects of brewing to focus on, it can become very easy to put something as seemingly unexciting as water chemistry on the back burner. Throw in some math, brewing salts, spreadsheets, ions, ratios, acids, and pH into the mix, and it can all feel overwhelming. Will applying all this extra effort into the beer really make a difference?
I asked myself this question recently while designing a recipe for one of my favorite styles, New England IPA. I began to dissect the style in my head and asked the question— what makes a NEIPA a NEIPA? The grist usually contains pale malt with a decent percentage of flaked adjuncts such as oats or wheat, which are used to add body to the beer, impart a slick mouthfeel, and aid in head retention. The hop choice usually consists of tropical, pungent, heady aromatic varieties. Then there’s yeast, which in the end will create some fruity, beautiful, often opaque happiness in a pint.
If all that isn’t enough, then there’s water chemistry.
A common recommendation when making NEIPA is to use water with a higher ratio of chloride to sulfate, often in the range of 2:1, though sometimes even higher. Unlike sulfate, which is known to accentuate hop bitterness and lend a drying effect to styles like American IPA, chloride allows the malt to come through, providing a fuller, rounder, smoother, and dare I say, juicy experience on the palate.
As someone who loves this style and brews it often, I couldn’t help but wonder if water chemistry really matters, whether the natural flavors from the malt, hops, and yeast really needed a boost from mineral and acid additions. My curiosity got the best of me as I contemplated all the possibilities, so I figured I’d test it out for myself.
| PURPOSE |
To evaluate the differences between a NEIPA brewed with treated water and the same beer brewed with untreated distilled water.
| METHODS |
I chose to use this New England IPA recipe of mine because of its delicious and easy drinking design.
Small Talk NEIPA
Recipe Details
| Batch Size | Boil Time | IBU | SRM | Est. OG | Est. FG | ABV |
|---|---|---|---|---|---|---|
| 4.7 gal | 60 min | 53.1 IBUs | 3.9 SRM | 1.056 | 1.014 | 5.5 % |
| Actuals | 1.056 | 1.01 | 6.1 % | |||
Fermentables
| Name | Amount | % |
|---|---|---|
| Pale Malt, 2-Row (Rahr) | 3.75 lbs | 40.54 |
| Pale Malt, Golden Promise (Thomas Fawcett) | 2.5 lbs | 27.03 |
| Wheat, Flaked | 1.5 lbs | 16.22 |
| Carafoam (Weyermann) | 12 oz | 8.11 |
| Oats, Flaked | 12 oz | 8.11 |
Hops
| Name | Amount | Time | Use | Form | Alpha % |
|---|---|---|---|---|---|
| Medusa | 42.5 g | 30 min | Boil | Pellet | 3.8 |
| Mosaic (HBC 369) | 21.3 g | 30 min | Boil | Pellet | 12.3 |
| Nugget | 14.2 g | 15 min | Boil | Pellet | 13 |
| Medusa | 42.5 g | 0 min | Boil | Pellet | 3.8 |
| Mosaic (HBC 369) | 21.3 g | 0 min | Boil | Pellet | 12.3 |
| Medusa | 71 g | 8 days | Dry Hop | Pellet | 4.8 |
| Mosaic (HBC 369) | 43 g | 8 days | Dry Hop | Pellet | 12.3 |
| Medusa | 70 g | 4 days | Dry Hop | Pellet | 4.8 |
| Mosaic (HBC 369) | 43 g | 4 days | Dry Hop | Pellet | 12.3 |
Yeast
| Name | Lab | Attenuation | Temperature |
|---|---|---|---|
| Juice (A38) | Imperial Yeast | 74% | 64°F - 74°F |
Notes
| Water Profile - treated: Ca 111 | Mg 5 | Na 8 | SO4 100 | Cl 150 Water Profile - untreated: distilled |
Download
| Download this recipe's BeerXML file |
The brew day started with the sound of water chugging out of one gallon distilled water containers into my Grainfather. As the Grainfather’s Connect controller beeped with each push of a button to achieve a set temperature, the coffee pot gurgled and filled the kitchen with the smell of freshly brewed java. Today was going to be a good day.
As the water was heating, I moved onto measuring out all brewing salt additions for the treated water batch including gypsum, calcium chloride, epsom salt, table salt, and lactic acid.
The mash water was then given a good stir to get everything thoroughly mixed. The untreated batch was brewed with the very same grains but received no brewing salts or lactic acid. The grains were then weighed out and milled for both batches.
Since I only own one Grainfather, the batches were brewed consecutively, starting with the treated water batch.

Both beers were mashed at 151˚F/66˚C. About 15 minutes into each mash, I took samples for pH measurement and found the treated batch hit my target while the untreated batch was slightly higher, predictably.

Following each 60 minute mash, I the grains were removed, a sparge was completed, then I brought the wort to a boil with the help of my HotRod heat stick.
While the wort was heating up, I prepared the hop additions, which I’d measured out the night before.
At the completion of each boil, I transferred the wort through a CFC to sanitized glass carboys.

Refractometer readings showed that the treated and untreated worts ended up with the very same OG.
I let the worts sit next to each other for a bit once in the carboys, at which point they looked identical to one another.

My yeast of choice was Imperial Yeast A38 Juice. I had heard nothing but good things about this strain and decided the experiment was as good of a time as ever to give it a try. Each batch received a single pouch directly pitched into the wort.
I saw signs of fermentation within 12 hours of the yeast being pitched and by day 3, the headspace in each carboy was filled with kräusen. At this point, I observed the treated water batch as being slightly more vigorous, at least by the amount of activity coming through the blowoff tube. Each batch was dry hopped on days 4 and 8 of fermentation.
Hydrometer readings showed the treated water batch ended with a final gravity of 1.010, while the untreated water batch finished at 1.007.

On day 10, the beers were cold crashed for 24 hours before being transferred to CO2 purged kegs and placed in my keezer.
I burst carbonated at 40 psi for 24 hours then reduced the pressure to 12 psi for 6 days of conditioning before the beers were ready to serve to participants.

| RESULTS |
A total of 16 people of varying levels of experience participated in this xBmt. Each participant was served 2 samples of the treated water beer and 1 sample of the untreated water beer in different colored opaque cups then asked to identify the unique sample. At this sample size, 9 tasters (p<0.05) would have had to identify the unique sample in order to reach statistical significance, though only 4 (p=0.83) made the accurate selection, indicating participants in this xBmt were unable to reliably distinguish a New England IPA treated to achieve a targeted water profile from one brewed with straight untreated distilled water.
My Impressions: My wife served me 4 blind triangle tests, and while the differences weren’t blatantly clear to me at first, I ended up picking the unique sample 3 times. In each case, I had to go back and forth between each sample multiple times before making my decision. I perceived the beer made with untreated water as brighter and more aromatic with a slight alcohol flavor and a touch more bitterness, while the treated water batch came across as rounder, smoother on the palate, and overall easier to drink. The downside of the treated batch was it seemed to lack the stronger aroma and flavor I picked up in the untreated batch.
| DISCUSSION |
Preference is a weird thing. When it comes to homebrewing, I view preference as being formed from comparison, familiarity, and/or experimentation. It can be as simple as comparing two finished beers and choosing your favorite. Maybe you liked the results of using a certain base malt so you never considered using something different. Perhaps every brew day is an opportunity for you to test out new methods and ingredients to dial in your process and get weird.
When it comes to brewing New England IPA, I have definitely developed preferences for certain malts, yeasts, and hops over time. I experimented with several different water chemistry profiles that led me to the conclusion that the 150 ppm chloride to 100 ppm sulfate tasted the best to me. I don’t know if it’s possible to have chloride sensitivity, but anytime I used a 2:1 ratio, I felt the beer lacked something, and the mouthfeel felt gritty, chalk-like. For a style that emphasizes hop aromatics, low bitterness, and tropical juice like flavors, I can’t help but wonder if the higher chloride is actually muting those desirable characteristics, hence the reason brewers tend to use such crazy amounts of dry hops, a tactic to achieve aroma while maintaining a full juice-like body.
While I definitely believe water chemistry brings something to the table, the fact tasters in this experiment couldn’t tell apart a NEIPA made with treated water from one made with untreated distilled water has me questioning its necessity. Both beers achieved a decent mash pH, had great aroma, and the flavors weren’t lacking. One observable difference between the beers is that the one made with treated water finished at 1.010 FG for an apparent attenuation of 81% while the one made with untreated water finished at 1.007 FG for an apparent attenuation of 87%, resulting in a nearly 1% difference in ABV. I’m not certain what actually caused this, but I thought it was surprising tasters were unable to select the unique sample in the triangle test based on the disparity in alcohol content alone.
This experiment has caused me to rethink my ideal NEIPA water profile. The brightness in aroma and flavor I perceived from the batch made with untreated water has stuck with me and made me question if my NEIPAs have been lacking in this area. Maybe dialing back on the chloride and pairing it in equal ratio with sulfate will help boost aromatics and flavor while still maintaining a silky smooth mouthfeel. Who knew this homebrewing thing would be such a slippery slope of questions and experiments with so few concrete answers?
| About The Author |
 Scott is a homebrewer from New Bedford, MA who is passionate about learning, brewing, and discovering styles he’s never heard of before. His obsession with homebrewing began in June 2017 and somehow he has managed to not only remain married, but he’s also a great dad. While his favorite style of beer to drink and brew is the all divisive New England IPA, he’s in fact a lover of all things IPA, Pilsner, and Stout. In hopes of refining his brewing process, he’s always in search of new ingredients, equipment, and methods to put to the test.
Scott is a homebrewer from New Bedford, MA who is passionate about learning, brewing, and discovering styles he’s never heard of before. His obsession with homebrewing began in June 2017 and somehow he has managed to not only remain married, but he’s also a great dad. While his favorite style of beer to drink and brew is the all divisive New England IPA, he’s in fact a lover of all things IPA, Pilsner, and Stout. In hopes of refining his brewing process, he’s always in search of new ingredients, equipment, and methods to put to the test.
Would you like to have your experiment featured on Brulosophy.com? Join The Brü Club today! The Brü Club is a growing community of curious homebrewers who regularly engage each other on topics important to us all. Membership is free and comes with all sorts of cool opportunities. Learn more at TheBruClub.com!
If you have any thoughts about this xBmt, please do not hesitate to share in the comments section below!
Support Brülosophy In Style!
All designs are available in various colors and sizes on Amazon!
Follow Brülosophy on:
FACEBOOK | TWITTER | INSTAGRAM
If you enjoy this stuff and feel compelled to support Brulosophy.com, please check out the Support page for details on how you can very easily do so. Thanks!






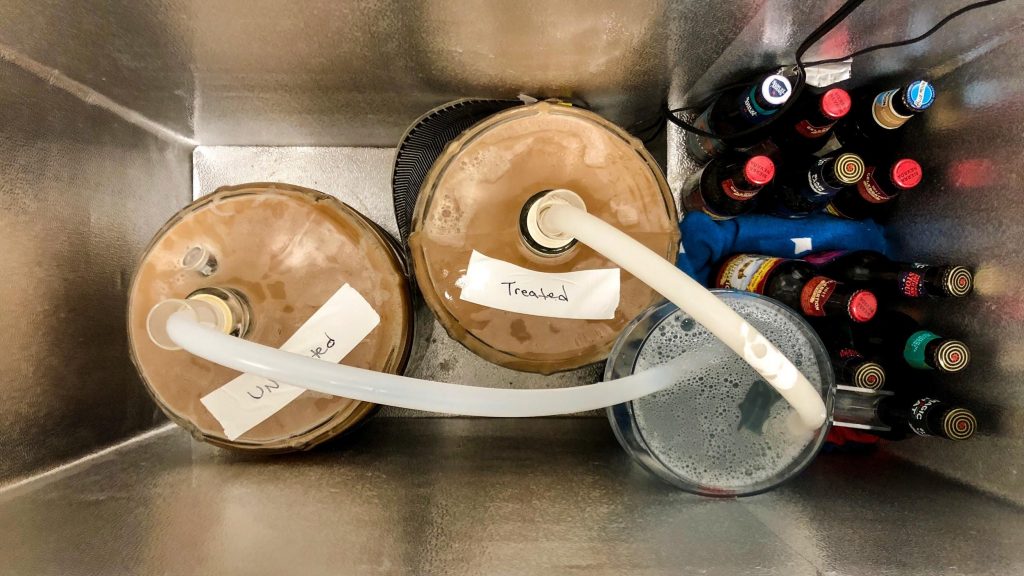











26 thoughts on “The Brü Club xBmt Series | Water Chemistry: Treated vs. Untreated In A Hazy IPA”
This is interesting. I think 5.2 is too low to be honest, and target that’s suited to commercial lager extract efficiency. Hop utilization is better at higher pH levels, but whether this means utilization of both alpha acids and hop oils, I’m not sure. Also, John Kimmich at The Alchemist isn’t one for the higher chloride, so I think the internet ‘lore’ around NEIPA doesn’t reflect the practices of the forerunners of the style, of which there’s quite a bit of variation. I mean, Tree House use S-04 as their main yeast :/
Awesome stuff. I love that no one could reliably tell the difference. My preference has trended toward higher sulfate with very soft water i.e.: low calcium and magnesium. Again, that’s just my personal preference as I can’t stand the gritty chalk-like beers. I always felt like I could use less hops overall and especially in dry hop with softer water and less chloride.
Great experiment!
I’m surprised the non treated batch had such a reasonably good mash ph. I was expecting to see a way higher ph without acid.
I think the main difference may simply come down to hop utilization being better with a higher ph. Before I started treating my water with acid and salts, I made awesomely aromatic, nasty tasting IPA’s.
Interesting theory. I too was surprised by how little the difference was with the ph. I believe past experiments have shown that small ph differences didn’t change much.Then again past water chemistry experiments have come back almost always significant. Beer can get weird.
Distilled water doesn’t have a pH of 7, it usually settles at around 5.8-6 as it absorbs carbon dioxide from the atmosphere to form carbonic acid.
I am also very surprised the distilled water batch with no acidification reached 5.4. Having very soft water in the Seattle area, 20 ppm hardness and alkalinity I would expect to see 5.7.
I may have missed it in the write up, but did you take the final pH of both beers?
I did not. I’ll keep that step
In mind for any future experimentation I do.
What type of tape are you using for you keg labels?
Just white duct tApe, sometimes silver
I’m a bit fed up with this blog concluding that non-statistically significant results indicate that “tasters can’t tell the difference”. That’s not what a non-significant result means at all! A non-significant result means that you are unable to conclude with the requisite level of certainty (i.e., 95%) that tasters CAN tell the difference. That’s a very meaningful difference, especially in the context of this blog, which always uses very small sample sizes.
If you run an analysis with 10 samples, it’s very hard to get a significant result. If you run the same analysis with 1000 samples, you’re much more likely to get a significant result. This blog keeps saying, in effect, “based on our non-significant result from an analysis using 10 samples, this variable doesn’t make a difference, so you can safely ignore this variable when brewing” — which is, to be frank, wrong.
I get that it’s not very sexy to say, “based on our non-significant result, we are unable to say anything with certainty — maybe if our sample size was larger it would show that the variable has an effect, or maybe it doesn’t have an effect”. But that’s probably what you should be saying when you get a non-significant result, at least with your current triangle test study design (which is designed to test for differences, not equivalency).
Here’s a paper that the Brulosophy Statistical Methodology Department should read: http://www.indiana.edu/~kruschke/articles/Kruschke2012JEPG.pdf
It would appear you’re reading the results wrong. Now, this was an article written by a member of The Brü Club, not Brülosophy, so my response is in regards to what we actually publish.
We never say “based on our non-significant result from an analysis using 10 samples, this variable doesn’t make a difference, so you can safely ignore this variable when brewing.” Actually, we very often overtly encourage our readers NOT to interpret our findings this way.
What we do say is whether or not the tasters in a specific xBmt were capable of distinguishing the unique sample in a triangle test enough to say it was significant at the p-value threshold of 0.05.
No need to get up in arms, either way, as it’s just beer we’re dealing with here. If you don’t like the we, or other people, approach experimentation, I’d encourage you to do some experimenting yourself. It’s fun and, at least for me, very eye opening. Cheers!
Apologies if you got the impression that I’m up in arms! This is my inside voice. I love the blog and this is my way of showing it!
I overstated the way that you report your results for emphasis, but the fact is that your standard language for reporting non-significant results is wrong. For example, in your recent xBmt dealing with flaked vs. malted oats, Brian Hall stated that, “In the end, only 7 tasters (p=0.58) accurately identified the unique sample, indicating participants in this xBmt were unable to reliably distinguish a NEIPA made with flaked oats from one made with the same amount of oat malt”.
That’s simply incorrect. The fact that only 7 tasters accurately identified the unique sample only indicates that we cannot say with certainty whether tasters can reliably distinguish a NEIPA made with flaked oats from one made with the same amount of oat malt. The result does not indicate that tasters are unable to reliably recognize the difference. That was not the result of the test, nor was the test designed in a manner that could yield such a result. There’s a good chance that the non-significant result was caused by the small sample size (21) rather than from an equivalency between flaked and malted oats.
If you want to draw conclusions about equivalency, you should tweak your methods.
No apology necessary! You’ll be happy to know we’re currently working with 2 statisticians to adapt the way we report results. Preliminary, our sample size actual doesn’t appear to be the reason for the lack of significant results. More to come in a bit. Cheers!
I would add that the issue is not just present in the standard language, but also in the discussion. For example, in the xBmt on flaked vs. malted oats, Brian Hall referred to “the apparent lack of a perceptible difference”.
And, in a recent, non-significant xBmt on mash temperature, Jake Huolihan referred to “tasters’ inability to tell apart beers mashed at either 147°F/64°C or 164°/73°C”, and also said that, “neither the differences in alcohol nor sugar content led to perceptible differences in the finished beers”.
Those statements mischaracterize the nature of a non-significant result.
The stats are fascinating. However, I think you can flip this on its head and say that sometimes the significant results don’t indicate that MANY tasters can actually tell the difference. Particularly with larger sample sizes. For example if we had a sample size of 1000 and 500 tasters picked the unique sample then this would score very highly on significance but when you do the maths it would mean that only 25 % of tasters could actually pick the unique sample (with 50 % getting it wrong and a further 25 % just guessing right). So whilst a significant result it would still mean that three quarters of tasters couldn’t tell the difference! And statistically it is likely that my pallet is one of the the 75 %! Love this blog and look forward to see what marshalls statisticians can show us all…
Just to add to this. For most of the experiments on this site we can say with a good level of certainty that MOST people cannot tell the difference even though we can’t say whether anyone can reliably tell the difference.
So if you’re not a “super taster” then these single variables don’t matter enoyto tell the difference.
Interestingly 90 % of drivers believe they have above average driving skills. Maybe us beer aficionados are the same with our pallets…
That’s a pretty low mash pH considering the grain bill. Depending on how much LA you added I might even doubt the accuracy of that reading. That said, higher pHs are anecdotally associated with “maltier” profiles. Maybe worth trying a third brew where you add the salts but not the acid.
Thought the same on the grain bill. I can say, brewing almost exclusively German style beers, if I want my hefe to be crisp, I dial it in at 5.2. I’ve gone as high as 5.9 before with only one serious but odd result. I carb all my hefe’s at 4.0. The one with the 5.9 pH lost it’s carbonation in under an hour. Never experienced that before.
I do enjoy reading the material on this site,but I think MJC is missing the big picture. It’s not the small sample sizes for me but the fact that it’s only done one time. For any exbeeriment to significant it needs to be done multiple times.
High chloride levels have the effect of reducing alcohol production and yeast biomass (via competition with Magnesium uptake) in yeast when at high levels (150ppm+). This may explain the higher FG of your treated sample and why the untreated one has more hop aroma and flavour (a drier beer accentuates hop flavour over malt flavour and volatile alcohols certainly seems to carry aroma or increase its perception). These aren’t significant in your taste test but explains how you described your beers on your blind taste test
As much as I do enjoy reading your articles, I must say there has been a lot of non-significant conclusions (semantics aside) lately. Now I would rather that be the case than every experiment show a significant difference – it’s far easier to introduce an unknown variable by error that makes beers appear different that’s nothing to do with what your testing. However when your beers vary by a percent or more in alcohol and you can’t get a significant result then the question is, how useful are your tasting panel? I have brewed a beer with no salts then taken a sample and dosed it with either 150ppm Calcium Chloride, Sulphate or water when deciding which of any to add and the difference is night and day, to the point I enjoyed one and had to pour one away. Adding pre-fermentation can only introduce more variance due to the potential influence on yeast activity (evident by different FGs). Given the beers are clearly different by measure, is there any ideas you have to improve the survey response sensitivity?
Have you tested anything like the difference 150ppm makes post and pre-fermentation? It’s my experience that beers which get to settle (even for a short amount of time) show a stark difference to the exact beer that was dosed in the glass.
https://brulosophy.com/2016/01/21/investigating-the-bad-palates-argument-a-graphical-look-at-xbmt-performance-based-on-experience-level/
Hypothesis on the final gravity. Calcium helps yeast to floc. Maybe more/faster flocculation in the treated beer, whereas the yeast in suspension in the untreated were able to drop it a few more points?
As someone who writes for a living (and makes beer for a hobby), I have to say, I really LOVE the intro to this article. It is the perfect blend of information and grab your attention. Kudos. And yes, it is also very informative, so there’s that as well. Thank you.