Author: Jake Huolihan
My grandpa’s swill of choice was always Miller Genuine draft, whether working around the house, out to dinner, or at family gatherings, you could find a MGD in his hand. It’s a fine tasting beer on its own, but he always did something a little unique by adding a pinch of salt before drinking it, explaining it “brings out the flavors.”
Used around the world as a general flavor enhancer, table salt (NaCl) is an ionic compound consisting of equal amounts of sodium (Na) and chloride (Cl). While both of these minerals are discussed when it comes to brewing water, chloride gets much more attention than sodium, though both purportedly contribute to a rounder malt character in beer. Whereas minerals like chloride and sulfate can be used in relatively high amounts, it’s generally recommended to keep sodium levels below 150 ppm to avoid salty flavored beer.
Out with my brother over the holidays, we both paid homage to our departed grandpa by adding a pinch of salt of our Miller Lite and raising a glass to him. It wasn’t necessarily an epiphanous moment, the beer didn’t taste all that different, but it did make me wonder the extent to which sodium has an appreciable impact on beer when used in higher amount in the mash.
| PURPOSE |
To evaluate the differences between pale lagers of the same recipe made with varying amounts of sodium in the mash.
| METHODS |
Wanting to go with a beer that was light in flavor to emphasize any differences caused by the variable, I whipped up my simple pale lager that was inspired by Anchor California Lager.
Like A Sailor
Recipe Details
| Batch Size | Boil Time | IBU | SRM | Est. OG | Est. FG | ABV |
|---|---|---|---|---|---|---|
| 6 gal | 60 min | 39.2 IBUs | 3.6 SRM | 1.051 | 1.014 | 4.9 % |
| Actuals | 1.051 | 1.012 | 5.1 % | |||
Fermentables
| Name | Amount | % |
|---|---|---|
| Pale Malt (2 Row) US | 11.75 lbs | 100 |
Hops
| Name | Amount | Time | Use | Form | Alpha % |
|---|---|---|---|---|---|
| Hallertau Magnum | 20 g | 60 min | Boil | Pellet | 11.5 |
| Loral | 30 g | 10 min | Boil | Pellet | 10.3 |
Yeast
| Name | Lab | Attenuation | Temperature |
|---|---|---|---|
| Cablecar (L05) | Imperial Yeast | 73% | 55°F - 65°F |
Notes
| Water Profile - Low Sodium: Ca 118 | Mg 0 | Na 8 | SO4 97 | Cl 140 Water Profile - High Sodium: Ca 41 | Mg 0 | Na 100 | SO4 97 | Cl 140 |
Download
| Download this recipe's BeerXML file |
I prepared a large starter of Imperial Yeast L05 Cablecar a few days ahead of time.
The day prior to brewing, I collected 2 sets of the same volume of water for each batch then proceeded to make the mineral adjustments. Using the Bru’n Water Spreadsheet, I determined the amount of table salt needed to add 100 ppm sodium to one batch while keeping the other at my typically low levels.
Note: since table salt also adds chloride ions, its use in the high sodium batch produced a difference in that particular mineral. Since chloride has been shown to have a perceptible impact on beer, I relied on calcium chloride (CaCl) additions in the low sodium water to balance the chloride levels, which led to a difference in calcium levels between the batches. After consultation with the Brülosophy crew, this was deemed the lesser of two evils.
I woke up the next morning and got started by turning on my heating elements then milling the grains while the water warmed.
Once the water reached strike temperature, I mashed in on the low sodium batch then did the same for the high sodium batch 20 minutes later, checking each to ensure I hit my target mash temperature.

Both mashes were left to rest for 60 minutes with occasional stirring.

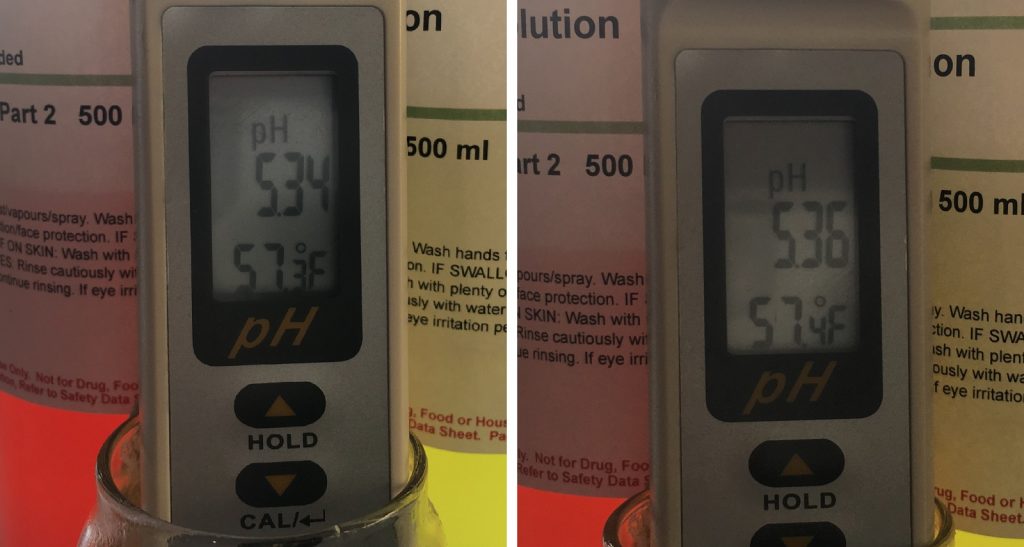
About 15 minutes into each mash, I pulled samples to check pH, which were right in line with what Bru’n Water predicted.
With the mashes complete, I removed the grain bags and brought the worts up to a rolling boil.

After each 60 minute boil, I quickly chilled the worts to just above my chilly groundwater temperature.

After racking equal amounts of wort from either batch to separate carboys, I took hydrometer measurements showing both worts had reached the same OG.

Each batch was then pitched with an identical amount of yeast from the decanted starter then hit with 90 seconds of pure oxygen. While both beers were actively fermenting 24 hours later, the low sodium batch appeared much more vigorous.
With signs of fermentation absent 7 days later, I took initial hydrometer measurements showing FG had been reached; follow-up measurements 3 days later were unchanged.

Skipping the gelatin fining process, I proceeded to keg the beers.

After a month of lagering on gas in my cool keezer, both beers were clear, carbonated, and ready to serve.

| RESULTS |
A total of 29 people of varying levels of experience participated in this xBmt. Each participant was served 2 samples of the low sodium beer and 1 sample of the high sodium beer in different colored opaque cups then asked to identify the unique sample. While 15 tasters (p<0.05) would have had to identify the unique sample in order to reach statistical significance, 16 (p=0.013) were capable of doing so, indicating participants in this xBmt were able to reliably distinguish a beer mashed with low sodium from one mashed with a higher amount of sodium.
The 16 participants who made the accurate selection on the triangle test were instructed to complete a brief preference survey comparing only the 2 beers that were different. A total of 7 reported preferring the low sodium beer, 7 liked the high sodium beer more, and 2 reported having no preference despite noticing a difference.
My Impressions: From the first sample at kegging to my latest pour, I could easily distinguish these beers, not only by taste, but aroma as well. In 5 blind triangle test attempts, I identified the unique sample every time without struggle. To me, the high sodium beer had a cleaner more pronounced hop aroma with sharper flavors and crisper mouthfeel than the low sodium beer. To my surprise, I preferred the high sodium beer.
| DISCUSSION |
With the propensity for water chemistry adjustments to have a noticeable impact on beer as observed in numerous past xBmts, I’ve admittedly come to expect comparisons of different mineral concentrations to produce distinguishable difference, hence my lack of surprise with the fact participants in this xBmt were able to tell apart a beer mashed with 100 ppm of sodium from one mashed with a more standard 5 ppm.
What I did find surprising was how great the high sodium beer tasted. Despite being under the 150 ppm max recommended by water chemistry experts, I really thought I’d be able to perceive the high sodium beer has being at least a little more salty than the low sodium beer, but it wasn’t at all. I not only perceived it as being crisper with sharper flavors, but half of the participants who were correct on the triangle test agreed with my preference for that sample.
Prior to this xBmt, I rarely considered using table salt in my brewing, focusing primarily on gypsum and calcium chloride for mineral adjustments like I tend to think most brewers do. Based on my experience with these beers, I’ll certainly be considering sodium levels in my brewing and look forward to exploring the impact it has at various levels in other styles of beer.
If you have any thoughts about this xBmt, please share them in the comments section below!
Follow Brülosophy on:
FACEBOOK | TWITTER | INSTAGRAM
If you enjoy this stuff and feel compelled to support Brulosophy.com, please check out our Support page for details on how you can very easily do so. Thanks!














60 thoughts on “exBEERiment | Water Chemistry: Impact Sodium Has On A California Common”
Your table salt has iodine?
No
You can get it either way. With iodine and without.
Is that means that ion exchange resin water softeners can be beneficial? Up to date I thought they were harmful. Mine works by removing Ca and Mg (I consider that a bad thing) and exchanging them for Na (I considered this bad/neutral) so I never used my softened water for brewing.
I don’t have a water softener so I wouldn’t really know from experience. It’d probably be good to know what the levels of Na are though prior to brewing with it. I only used 100 ppm here and it was very noticeable imo.
Hey that is very interesting! I just moved to a new apartment and there’s one of these water softeners in use in my building. I was strongly advised against using my softened tap water for brewing…however after reading this, I might feel adventurous and give it a try! The water is only partially softened. They probably blend the softened with the original water either to reduce costs and/ or to keep Na to a moderate level. Anyway there is definitely still some significant amount of Calcium in there, and I cannot taste even the slightest hint of saltiness in it. So I guess that the Na level is probably not exceeding the ca. 100 ppm level. Anyway I’m intrigued…think I’ll give it a try while of course reducing the alkalinity through acid and also adding some gypsum and CaCl
Did you use pure NaCl?
At least here in Europe table salt often contains Sodium ferrocyanide as anticaking agent, which could be poisonous for the yeast. Maybe you got a slower ferment because of this. Iodine or flour if present in the salt could also be a reason.
Really doubt that any of the additives to the table salt would cause any issues for the yeast considering the very small amounts added to the beer. Potentially lower Ca levels could cause the yeast to grow slower since calcium is very important for many biological processes.
Yes pure NaCl
Would be interesting to see a test with the same Ca levels as well, you could try to use Sodium Phosphate, or Sodium lactate instead of NaCl.
I would also like to repeat this while keeping calcium equal. I’ve personally never used sodium lactate so I’m unsure what the dissolved form of that would be in water but I would assume some form of bicarbonate looking at the formula?
In terms of phosphates I’ve used those before I believe as the five star product 5.2. At the end of the day there will always be a secondary variable when you start messing around with these different ions. I’m fairly interested now if calcium can be identified independently in beer.
We’ll lactate would be lactate, the same as in lactic acid. I suggested it as lactic acid is said to be quite taste neutral 🙂
I think the experiment is perfect as is. There is a lot of information online that says using kosher salt in your beer is a bad idea. But years of cooking tells you salt make things taste better. I put a teaspoon of salt in the mash of a 5 gallon batch of IPA the other day. Your results are encouraging. Can’t wait to see how mine turns out.
Yes he could have found some other hard to find alternative as a way of adding sodium but we all have salt in our homes. Should we try using it? The answer is YES! Awesome experiment!
Interesting result. I think what this exbeeriment shows is that there’s more to mineral levels than just the taste of the minerals themselves. Minerals could be interacting with various chemical or biological processes that affect the beer’s final flavour, from yeast metabolism and ester production to hop utilization, biotransformation, and other processes we’re unaware of. All the more reason to take care with water chemistry.
Yeah the water Chem series of xbmt have opened up way more questions than answers for me
I found out the hard way what happens when I use the soft water from the winery. I ended up with a beer that had a sharp bitter after taste. The sodium ion exchange in the softened water accentuated my hops and made for a rather unpleasant Munich Dunkel beer.
To my belief, you may add a pinch of salt to low hops beers like Miller, and others, but you will really not like it in a very hop forward IPA beer, and especially if you are sensitive to too much bittering.
Vern
Sodium reduces the bitterness of foods. That’s why it’s common on grapefruit and in coffee for some people.
https://www.quora.com/Food-Chemistry-How-does-salt-make-coffee-less-bitter
https://www.nih.gov/news-events/nih-research-matters/high-salt-detected-sour-bitter-taste-cells
https://www.scientificamerican.com/podcast/episode/too-much-salt-gets-bitter-and-sour-13-02-13/
As with any water adjustments, each recipe has its own quirks and trial and error is often the best indicator (small batches) of good/bad results.
I routinely adjust all my beers with CaCl, Gypsum, Epsom and some form of course sea salt and SMB. I’ve never had a perceived mineral issue but I check ppm for each mineral and I only use enough to get around 100ppm Ca and obtain PH with city water at about 8.1.
To be even more unconventional, I also use small salt and acetic acid (white vinegar) adjustments in my sparge water because.. I’m cheap. And since first doing it I’ve never noticed a change in flavor except that I can over sparge without risking tannin extraction due to my sparge water PH being around 5.5.
Another way to add sodium: sodium bicarbonate, baking soda with excess bicarbonate neutralized with acid addition.
Absolutely! But does bicarbonate have a flavor impact?
If lactic acid is used to neutralize the bicarbonate that suggestion would give the same result as adding sodium lactate directly. The bicarbonate would leave as carbon dioxide so would not impact any flavour.
Awesome, I’m not familiar with this acid looks like it’s sold in 60% concentration on Amazon. I’ll look into it.
I would be interested to see if a higher level of sodium would have the same positive effect on a more hop-forward beer such as an APA or IPA. Thanks for all you guys do!
Same here!
I already did the test working between 50 and 75 ppm of NaCl, the result was a more pronounced profile in the matter of taste / bitterness
Yes indeed, sodium is a great flavor enhancer if not overdone. I always put a little in my French Roast coffee as well. People love it. Kosher or Himailan salt with no iodine. I have been adding a teaspoon to 10 gallon malty beer mashes along with Gypsum & CaCl to get water where I want with about 100 ppm Ca. I’m fortunate to have VERY soft Alderwood artesian well water to start with. 86 Bicarbonate, everything else super low. I stay away from Chalk & Baking Soda because they both RAISE pH. Acidify all brewing water to 5.5 pH per practice by Sierra Nevada & Gordon Strong. Works well for me!
I would bet the quicker vigorous ferment of low sodium batch was due to almost THREE TIMES the Ca that was needed by the yeasties.
I would agree the more vigorous ferment was likely a combination of increased calcium and potentially the sodium. Many baking books I have talk about bumping up salt to suppress fermentation.
fascinating xbrmt. I have always added 2 or 3 green olives to a miller lite. It is my guilty pleasure at neighborhood restaurant. They call it a townie martini… I now realize it may have been the salt from the olives that made the miller lite taste better.
A fantastic combination!
Following up on Anders comments, have you thought about using sodium phosphate or using potassium in the control (eg, KCl vs NaCl); if yes, why did you discard them as options? Both phosphates and K are high in wort/ beer so an addition is not likely to have a large effect. Conversely, calcium appears to have an effect on flavor, either directly or binding to proteins, masking their flavor. I am sure you weighted pros and cons, so I would like to see how the decision-making process was.
Thanks for doing the experiment 🙂
Mainly I considered NaCl and NaHCO3 for this xbmt since both are commonly used by homebrews, both also contribute a secondary variable. I’ve personally never heard of calcium affecting flavor in beer, in fact the opposite, do you have a source on calcium as a flavor ion?
The only other time I’ve tasted beers beers where one calcium level was much higher than the other was in this xbmt https://brulosophy.com/2017/05/01/water-chemistry-pt-8-the-impact-of-mineral-load-when-ratio-is-the-same-exbeeriment-results/ and those tasted identical.
Definitely interested in trying sodium lactate since it would be easier to equalize given pure lactic can simply be added to the corresponding mash.
Cheers
What would be the consequence of adding NaCl to a tap water without modifying anything else ? I guess since Cl- ions are added, sulfate/chloride ratio gets lower and the hops are less pronounced. That would be contradictory to what you show here with hop getting accentuated with increase of Na+. Do you have idea which ion would “win” ? Na+ or Cl- ? Thanks for the experiment !
Yeah I honestly don’t know. Only one way to find out!
My yellow fizzy water of choice for years has been a coore’s light with salt and a generous squeeze of lime. That salt trick on grapefruit is no joke either. I remember Alton Brown saying that salt fills a receptor in the taste buds reserved for bittering; leading to a sweeter flavor.
Really interesting experiment!
I love coors, will try a hint of lime next time I have one
The Alton Brown episode is what I was looking for in regards to the bitterness receptors. It’s thought to be a safety mechanism so we don’t over consume salt. Or just the chemistry set known as the human condition. 😀
One of the reasons I love Brulosophy is because it reminds me of Good Eats…. use science to challenge the conventional wisdom. 👍👍
Another strange thing… salt is the only “rock” that humans must eat to survive. I can see that as one reason we love it so much.
Great xBmt, as usual. I switched from CaCl to Kosher Salt as my chloride source in my lagers a few years back, targeting the 40-50ppm range for sodium, and there was a noticeable improvement. I compare it to forgetting to salt your pasta water. It’s good to see others get similar results, and good to know that there’s considerable headroom above the levels I normally target.
I will be playing with higher levels in the near future, and may start including this in my ale brewing water as well.
Speaking of water chemistry…. some old questions on this one:
https://brulosophy.com/recipes/whatre-we-here-for/
Interesting. Could this be a difference in lower Ca?
It definitely could be causing some of the difference, really no way of knowing.
Interestingly enough this xbmt: https://brulosophy.com/2017/05/01/water-chemistry-pt-8-the-impact-of-mineral-load-when-ratio-is-the-same-exbeeriment-results/
Did not achieve significance with even a larger calcium delta.
And that is a massive design flaw in your experiment. You have no idea which variable change resulted in a flavour change, especially considering the importance of calcium in brewing and the low levels present in the high sodium beer. You can’t pick and choose here, that’s why you need to design an experiment that alters only one variable at a time. Another meaningless result.
I don’t think it’s a design flaw since it was known going into it. Basically you have to choose which secondary ion will be higher when you alter sodium levels. We could have gone with an increased bicarbonate level, increased chloride level, increased phosphate level, etc. elemental sodium cannot be added straight to the water profile to make it be the only variable. Since calcium had not indicated potential for previous flavor impact, and common knowledge says that it’s not a flavor ion we chose that. Again ideally we’d love to keep sodium the only variable, however it’s simply not practical.
It’s a design flaw whether you think it or not. You can not draw any conclusion from this experiment due to the nature of its design, the fact that you are aware of this makes it worse. You have really just wasted your time as it means nothing. Sorry for being so harsh as I admire the premise of what you are trying to achieve, but this is by and far one of the worst experiments I’ve read so far.
This could be like chasing down a long code. You’d have to have a base recipe, grains, water, hops, yeast, then just change the mineral content.
It’s like replacing multiple components on an engine or PC and then, it’s fixed… what was broken? Negative feedback regarding the methodology of these experiments could lead to better experiments.
“To infinity and beyond!”
-Buzz Lightyear
It’s not possibly to make sodium the only mineral variable, at least not with means available to me.
Everything else in this xbmt was kept constant: grains, water, hops, yeast. The only variables were ppm of sodium and calcium
Then you cannot perform this experiment in a manner that will allow a conclusion to be made. That in itself isn’t really a problem, just accept the limitations of what variables you can and can’t experiment with at any given time and work within those limitations. All scientists have to do the same (or find a ton of cash to fund what they want to do).
The criticism from Adam is valid. Your experiment compares Ca to Na and it should probably have been more clear, but not all is lost given you found a difference; which can be attributed to sodium, calcium or the combination, and you now need to do more experimentation to explain it. You do need something to balance sodium, and my suggestion would have been phosphate. Phosphate is a better choice because it has a negligible flavor impact, and also because there is a lot of phosphate from the malt already, so the difference in phosphate between one beer and the other is minimal. Keep experimenting 🙂
Hm I felt the differences were very clearly stated in the article, oh well I’ll keep that in mind for next time.
So you’re claiming calcium does have a flavor impact and that excess phosphates don’t? Any sources on this, interested?
I’ve seen a paper in Nature about this. Google Calcium taste receptor.
I was referencing the single (constant) recipe in regards to:
“It definitely could be causing some of the difference, really no way of knowing.
Interestingly enough this xbmt: https://brulosophy.com/2017/05/01/water-chemistry-pt-8-the-impact-of-mineral-load-when-ratio-is-the-same-exbeeriment-results/
Did not achieve significance with even a larger calcium delta.”
In terms of the Ca, if you use this experiment and compare it to the calcium levels to an experiment with a totally different recipe that could add to variables in the outcome, no?
https://www.scientificamerican.com/article/osteoporosis-calcium-taste-chalk/
https://www.livescience.com/5059-sixth-taste-discovered-calcium.html
I’m sure this varies from person to person.
So if you now add the equivalent salt to the remaining low salt beer do they become indistinguishable. If you have enough left over the next experiment only takes a few seconds. If so then it becomes logical to adjust salt post brew to get the flavour you want like your dad did. More control and less risk.
I just moved from an area with a reasonable sodium concentration in the water to an area with basically no ionic content in the water and have definitely noticed a change in previously tried and true recipes. I’d actually narrowed down on the sodium so I’m glad to see some supportive evidence here
I would try an experiment with MSG instead of NaCl. =)
The other day i was brewing a Pale Ale, with a relatively minerally water profile.
In other to get a the right mash pH I added lactic acid; however i miscalculated the amount of lacic acid and I ended up with a mash pH of 4.7.
To get back to the 5.2-5.4 range I added Sodium Bicarbonate (Baking Soda). Thus, I ended up with high amount of Sodium in my mash.
As the experiment describes the beer came out with great hop flavor and crispness.
Prior to reading this Experiment I couldn’t tell why the beer was so flavorful
I appreciate this article so much. I did a NEIPA yesterday where I used NaCl in my water to add Cl in the water chemistry.
Sweet, let us know how it turns out
Excelente información